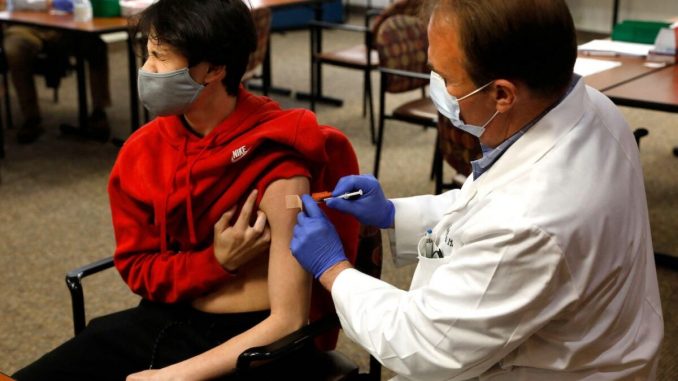
In the United States, around 600,000 children aged 12 to 15 have been vaccinated against COVID-19 as of Tuesday, according to the U.S. Centers for Disease Control and Prevention.
CDC Director Rochelle Walensky said in a media call that the figures come after the COVID-19 vaccines from Pfizer-BioNtech became the first to be cleared for that age group in the United States earlier this month.

The Food and Drug Administration (FDA) had declared on May 10 that the Pfizer vaccine is safe and offers strong protection for younger teens, based on testing of more than 2,000 U.S. volunteers aged 12 to 15. Two days later, the CDC officially recommended the shot for the age group.
President Joe Biden has asked states to make the vaccine available to younger adolescents immediately. Most states began issuing shots to children last Thursday but some, including Georgia, started sooner.
“Now it’s time to get your shot. We have the vaccine. We’ve secured enough supply to vaccinate all adults and children above the age of 12. I repeat: Now is the time to get your vaccine shot,” the president said on May 17 in an update on the federal vaccination program.
The Pfizer-BioNTech vaccine has been available under an emergency use authorization to people as young as 16 in the United States since December.
The CDC acknowledges that fewer children have been infected with the CCP (Chinese Communist Party) virus compared to adults. The agency notes, however, that children can still be infected and get stick from the CCP virus, as well as spread it to others.
As of May 15, its website issued a message recommending “everyone 12 years and older” to be vaccinated “to help protect against COVID-19,” characterizing widespread vaccination as a “critical tool to help stop the pandemic.”
“Getting your child or teen vaccinated can bring you one step closer to enjoying the activities you miss. Children 12 years and older are able to get the Pfizer-BioNTech COVID-19 vaccine,” the CDC message reads.
Vaccine manufacturers are immune from liability for any adverse reactions unless there is “willful misconduct” involved. Vaccine providers are required to report any serious adverse effects or vaccination administration errors to VAERS, hosted by the U.S. Department of Health and Human Services.
The federal government has a Countermeasures Injury Compensation Program that can pay compensation to eligible persons who suffer serious injury from approved vaccines. But the burden of proof has proven a challenging process.
Reuters contributed to this report.






Be the first to comment