OAN Staff James Meyers
11:10 AM – Tuesday, October 22, 2024
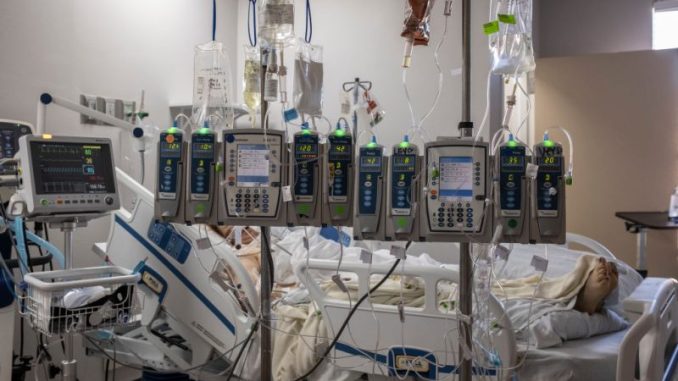
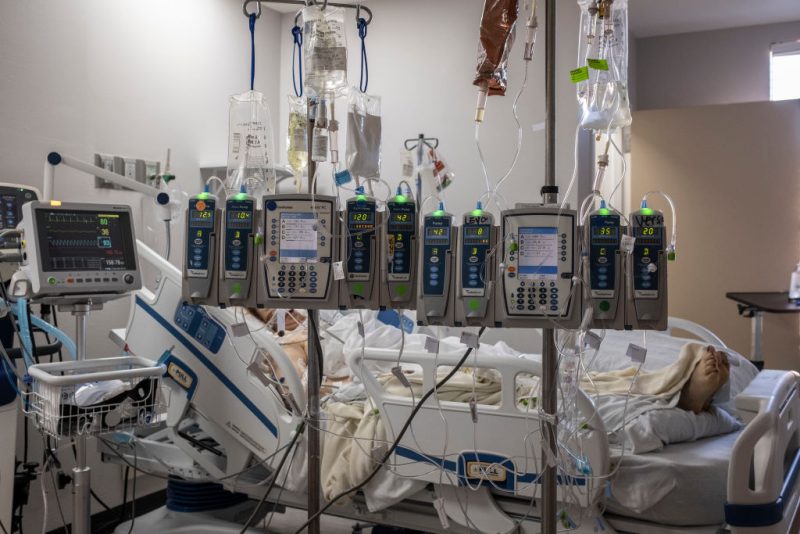
Multiple hospitals across the United States are reportedly dealing with major issues related to a shortage of IV fluid after flooding from Hurricane Helene forced medical fluid manufacturers to halt production.
Advertisement
“A plant involved in producing IV solutions for one of the country’s largest manufacturers was flooded as a result of Hurricane Helene,” the BBC reported.
Baxter International, the leading supplier of IV fluids to hospitals, said that its facility in Marion, North Carolina, will remain closed for the foreseeable future.
“Baxter’s North Cove facility affected by flooding and currently closed for production as the company assesses extent of the impact,” the company says in a statement on its website.
IV fluids are used to deliver drugs or water with electrolytes directly into a patient’s bloodstream. They’re also critical for surgery, when a person is unable to eat or drink for any reason, keeping them hydrated. Baxter also makes specialty fluids, such as peritoneal dialysis fluid, which helps patients with kidney failure filter waste from their blood, as well as irrigation fluids used during procedures to clean or flush wounds.
“Remediation efforts are already underway, and we will spare no resource — human or financial — to resume production and help ensure patients and providers have the products they need,” José (Joe) E. Almeida, chair, president and chief executive officer at Baxter, said in a statement.
Additionally, Mass General Brigham is among the health care systems that has not received its usual supply of IV fluid. Chief preparedness and continuity officer Dr. Paul Biddinger said during a press conference on Friday that the hospital network expects to receive about 40% of what it usually gets from Baxter.
Biddinger called the supply constraint “one of the biggest shortages” the hospital network has ever dealt with.
Meanwhile, other manufacturers of IV fluids say they’re increasing production to help cover the shortage. B. Braun stated that none of its manufacturing sites were affected by Hurricane Helene, and that it’s “taking immediate steps to increase production at our pharmaceutical manufacturing sites in Irvine, California, and Daytona Beach, Florida, focusing on critical IV fluids.”
Furthermore, the company also placed its products on “protective allocation” and is now encouraging providers that administer IVs to practice conservation, which includes using alternate hydration methods.
In 2017, hospitals experienced IV fluid shortages when Hurricane Maria halted manufacturing.
“Having experienced similar challenges in the wake of Hurricane Maria in 2017, we continue to be mindful of how we manage the supply of these medications to ensure minimal impact on our patients. Hospital operations continue as normal and patient care remains unaffected,” Dr. Biddinger of Mass General Brigham said in a statement.
Stay informed! Receive breaking news blasts directly to your inbox for free. Subscribe here. https://www.oann.com/alerts
Advertisements below






Be the first to comment